SAN ANTONIO--(BUSINESS WIRE)--Sep 5, 2023--
The U.S. Food and Drug Administration (“FDA”) has granted Xenex Disinfection Services, Inc. (“Xenex”) De Novo authorization for the company’s LightStrike TM + device, a high-intensity, broad-spectrum ultraviolet (UV) light robot. The authorization creates a new medical device product classification under which the LightStrike+ robot is the first and only product of its type, setting the precedent for FDA regulation of UV robots intended for use in reducing pathogens on non-porous, high-touch surfaces in the healthcare environment.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230905128680/en/
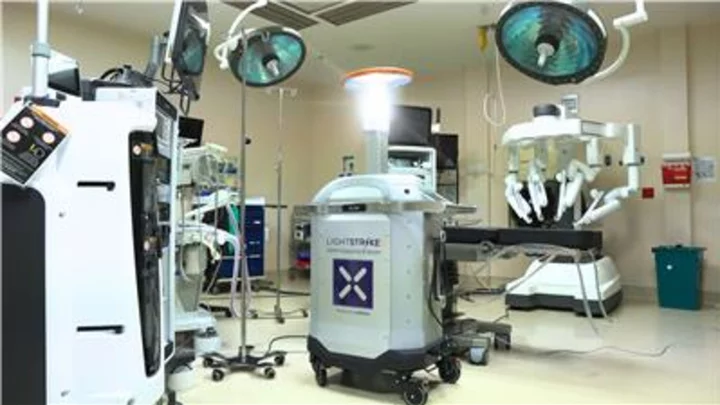
FDA Authorizes Xenex LightStrike+ UV Robot via De Novo – First & Only Microbial Reduction Robot for Healthcare Facilities. Sets precedent for FDA regulation of UV robots intended for use in reducing pathogens on non-porous, high-touch surfaces in the healthcare environment. (Photo: Business Wire)
LightStrike+ devices are intended to perform microbial reduction on non-critical medical device surfaces free from visual soiling in the healthcare environment following manual cleaning and disinfection practices. They are authorized for use in healthcare facilities, including unoccupied operating rooms, hospital rooms, and other clinical settings where non-critical medical devices may be present.
More than 1,200 healthcare facilities worldwide have run LightStrike robots over 37 million cycles. With as low as a 2-minute run-time for microbial reduction, the new LightStrike+ device is built upon accumulated knowledge from more than a decade of best practices implemented at healthcare facilities, 45 peer-reviewed studies demonstrating safe and effective use, 193 patents, and industry leading technical and epidemiological expertise. Many of the world’s leading hospitals, including HonorHealth, Mayo Clinic, MD Anderson Cancer Center, Ochsner Health System, Stanford Health Care and Texas Health Resources use LightStrike robots as part of their comprehensive disinfection strategy.
Dangerous pathogens remain on surfaces in healthcare facilities, even after thorough manual cleaning efforts. The LightStrike+ robot uses a xenon lamp to create high-intensity pulsed UV light that reduces the number of those pathogens on surfaces and helps to break the chain of transmission from one patient or healthcare worker to the next. Xenex’s FDA authorization is supported by rigorous testing performed on over 10,000 samples of vegetative bacteria and Clostridiodes difficile (C.diff) spores.
“We have been working with our hospital partners for over a decade to support them in their mission to improve patient safety and public health. As an infectious diseases epidemiologist, I am highly concerned about antibiotic resistance in the hospital environment. I hope that FDA authorization will allow hospitals to more easily use this tool in their fight against pathogens,” said Dr. Mark “Tuck” Stibich, founder and Chief Scientific Officer of Xenex.
“We understand that it can be challenging for hospitals to evaluate UV technologies, especially given the unverified and often exaggerated claims made by some manufacturers. FDA authorization gives hospital decision-makers the confidence that Xenex’s claims for LightStrike+ are accurate and validated,” said Xenex Chief Executive Officer Morris Miller.
Manufactured in San Antonio, Texas, LightStrike+ robots are now available.
About Xenex Disinfection Services
Xenex is a world leader in innovative UV technology-based strategies and solutions. Xenex's mission is to enable its partners to save lives and reduce suffering by destroying the deadly microorganisms that can cause infections. Xenex is backed by well-known investors that include EW Healthcare Partners, Piper Sandler, Malin Corporation, Battery Ventures, Targeted Technology Fund II, Tectonic Ventures and RK Ventures. For more information, visit xenex.com.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230905128680/en/
CONTACT: Melinda Hart
Xenex
210 240 4669
Melinda.hart@xenex.com
KEYWORD: UNITED STATES NORTH AMERICA TEXAS
INDUSTRY KEYWORD: TECHNOLOGY INFECTIOUS DISEASES HOSPITALS FDA OTHER HEALTH HARDWARE MANAGED CARE ROBOTICS HEALTH DATA MANAGEMENT
SOURCE: Xenex Disinfection Services
Copyright Business Wire 2023.
PUB: 09/05/2023 07:00 AM/DISC: 09/05/2023 07:02 AM
http://www.businesswire.com/news/home/20230905128680/en